Under clinical observation: hybrid ceramics on implants
In the context of a multicenter clinical application study over a maximum observation period of four years, a survival rate of 98.7 percent was determined for VITA ENAMIC crowns on implants. 38 patients were treated by dentists from Germany, Austria and Switzerland with a total number of 60 crowns during this period of time. The average period of intra-oral wear at the time that the report was written was 23.1 months. Master dental technician Claus Pukropp explains the main objectives and results of the investigation.
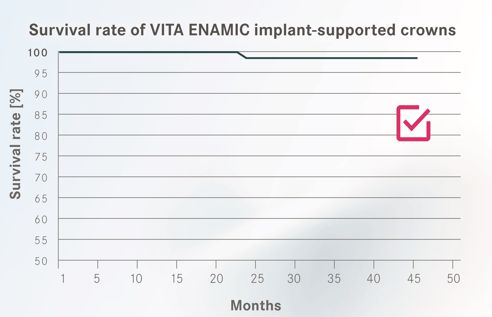
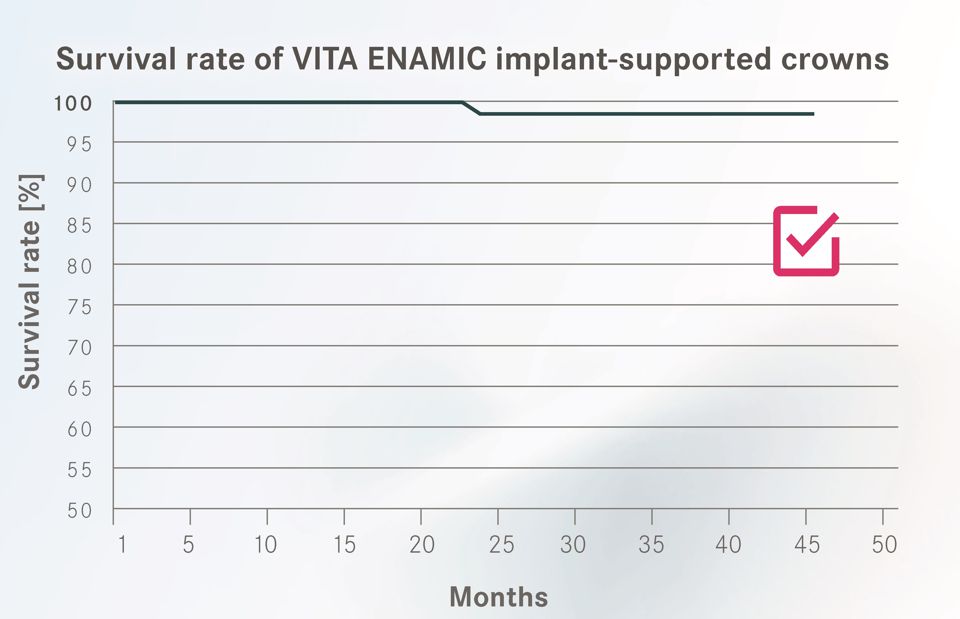
The objective of the investigation was to prove the suitability of the hybrid ceramic VITA ENAMIC for implant-supported crown restorations. To determine the survival rate (Fig. 1), debonding of the crown and partial or full fracturing (chipping) of the crown body was assessed as a loss criterion.
Areas of the jaw restored and types of restorations performed
In this clinical observation, the implantsupported restorations were done in the maxilla 41 percent of the time and in the mandible 59 percent of the time. 91 percent of the reconstructions examined were located in the mainzone of chewing pressure (premolar and molar area). A total of eight different implant systems were used. The majority of implant abutments were manufactured as follows: In 90 percent of the cases, individual abutments or monolithic abutment crowns of VITA ENAMIC on TiBase adhesive bases (72 percent) or alternative titanium bases (18 percent) were fabricated. The determined data on the implant-supported restorations therefore follow the trend, recognized in the literature, towards CAD/CAM-manufactured and screw-retained solutions.
An initial summary
The results of the study demonstrate comparable or higher survival rates1-3 for VITA ENAMIC implant crowns than for alternative materials. Over the entire observation period, only one crown fracture (after 25 months) was documented. This fracture could be attributed, according to information from the dentist, to a significantly thinner wall than the recommended minimum wall thickness (1.0 mm occlusal). In combination with the absorbing and damagetolerant properties, the initial clinical results for VITA ENAMIC implant-supported crowns allow us to expect long-term durability.
Report 09/15
References
1) De Boever AL, Keersmaekers K, Vanmaele G, Kerschbaum T, Theuniers G, De Boever JA. Prosthetic complications in fixed endosseous implant-borne reconstructions after an observation period of at least 40 months. J Oral Rehabil. 2006 Nov;33(11):833-9.
2) Thoma DS, Brandenberg F, Fehmer V, Büchi DL, Hämmerle CH, Sailer I. Randomized Controlled Clinical Trial of All-Ceramic Single Tooth Implant Reconstructions Using Modified Zirconia Abutments: Radiographic and Prosthetic Results at 1 Year of Loading. Clin Implant Dent Relat Res. 2015 Apr 15.
3) Rinke S, Lange K, Roediger M, Gersdorff N. Risk factors for technical and biological complications with zirconia
Source fig. 1: Multicenter clinical application study; VITA Zahnfabrik, Claus Pukropp et al., Bad Säckingen, Germany, 11/2014
Note fig. 2: Study report published in the technical and scientifi c documentation for VITA ENAMIC, VITA Zahnfabrik, Bad Säckingen, Germany